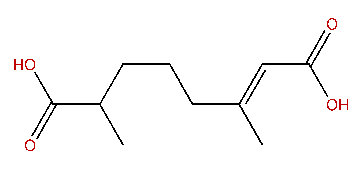
| (E)-3,7-Dimethyl-2-octene-1,8-dioic acid |
| Formula: | C10H16O4 |
| CAS#: | |
| MW: | 200.23 |
[MS]
[ NMR ]
[Behavioural function]
|
|
Reference(s) for synthesis of (E)-3,7-Dimethyl-2-octene-1,8-dioic acid [callosobruchusic acid]
| Fontana, E.A., Carpita, A., and Rossi, E. 1993. A simple synthesis of (R),(S)-(E)-3,7-dimethyl-2-octene-1,8-dioic acid, a copulation release pheromone component of the azuki bean weevil. Synth. Comm. 23:2797-2806. |
| |
| Gramatica, P., Giardina, G., Speranza, G., and Manitto, P. 1985. Baker's yeast hydrogenation of carbonyl activated double bonds. Enantioselective synthesis of the (S)-form of the dihydroterpenediol secreted by Danaus chrysippus and of a pheromone of Callosobrachus chinensis L. Chem. Lett. 1395. |
| |
| Mori, K., Ito,T., Tanaka, K., Honda, H., and Yamamoto, I. 1983. Synthesis and biological activity of optically active forms of (E)-3,7-dimethyl-2-octene-1,8-dioic acid (callosobruchusic acid). A component of the copulation release pheromone (erectin) of the azuki bean weevil. Tetrahedron. 39:2303-2306. |
| |
| Tanaka, K., Ohsawa, K., Honda, H., and Yamamoto, I. 1982. Synthesis of erectin, a copulation release pheromone of the azuki bean weevil, Callosobruchus chinensis L. J. Pest. Sci. 7:535-537. |
| |
| Wenkert, E., and Khatuya, H. 1999. Formal synthesis of (±)-callosobruchusic acid. Synth. Comm. 29:3051-3056. |
| |
| Yajima, A., Akasaka, K., Yamamoto, M., Ohmori, S., Nukada, T., and Yabuta, G. 2007. Direct determination of the stereoisomeric composition of callosobruchusic acid, the copulation release pheromone of the azuki bean weevil, Callosobruchus chinensis L., by the 2D-Ohrui-Akasaka method. J. Chem. Ecol.33:1328-1335. |
| |
|







